|
Introduction:
Spravato, also known by its generic name esketamine, is a nasal spray medication that has gained attention for its potential benefits with treatment resistant depression and suicidal thoughts. Spravato was approved by the United States Food and Drug Administration (FDA) in 2019, and it offers a novel approach with promising results in clinical trials. This article provides a comprehensive overview of Spravato, discussing its FDA-approved uses, benefits of use, potential side effects, and the Risk Evaluation and Mitigation Strategy (REMS) protocol associated with its administration. FDA Approval and Indications: The FDA approved Spravato for two main indications: treatment-resistant depression (TRD) and major depressive disorder (MDD) with acute suicidal ideation or behavior. It is intended for use in conjunction with an oral antidepressant and under the supervision of a healthcare professional, most commonly a psychiatrist. This approval marks a significant development in the treatment of depression, particularly for patients who have not responded adequately to traditional antidepressant therapies. Benefits of Use:
Side Effects: While Spravato holds promise as a treatment option, it is important to be aware of potential side effects. Common side effects associated with its use include:
REMS Protocol: Due to the potential for abuse, misuse, and adverse effects, Spravato is subject to a Risk Evaluation and Mitigation Strategy (REMS) protocol. The REMS protocol aims to ensure safe and appropriate use of the medication and includes the following elements:
Patient Experience: Once a patient has attended an initial consultation, your doctor will assess your condition and review your medical history. If Spravato is an appropriate treatment option for you, a personalized treatment plan will be developed based on your specific needs. Blood work will be done if necessary and followed up by staff. The treatment plan will outline the frequency and duration of your Spravato sessions. Typical treatment plans include 8 Spravato treatments over four weeks. During the initial start period, 2 doses will be administered 48-72 hours apart. After 4 weeks, 1 treatment per week followed by 1 treatment every 1-2 weeks as needed for maintenance. To prepare for your Spravato administration:
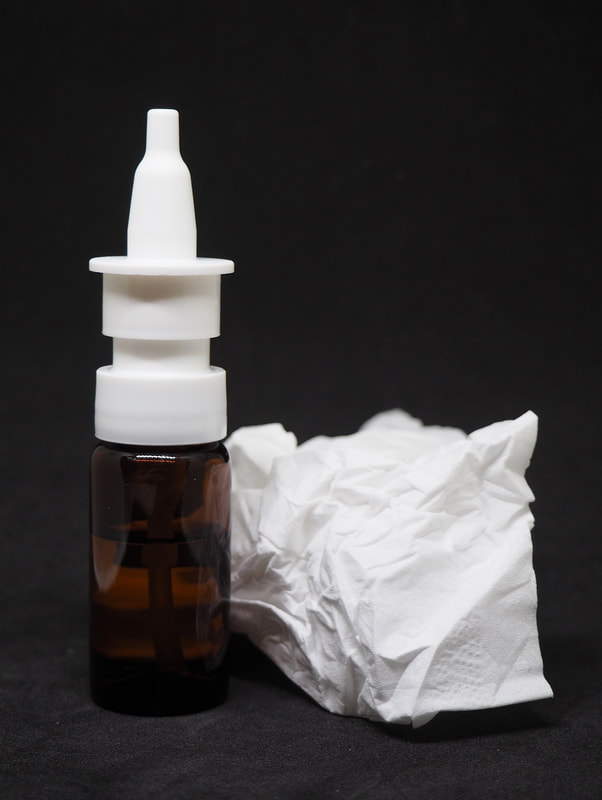
Upon arrival at Mynd Psychiatry, patients will receive a blanket and eye mask. Patients will be guided to the treatment room filled with relaxing chairs and footrests, low lighting, and peaceful music. This room is designed to allow patients an opportunity to relax and reach full immersion of Spravato while under the supervision of a healthcare professional. Patients will be encouraged to close their eyes and place the eye mask over their eyes during treatment to reach maximum immersion. A healthcare provider will be monitoring the room and available at all times for any needs patients may have. Spravato is self administered under the supervision of a healthcare provider. The healthcare professional will instruct you on the proper technique for using the nasal spray and guide patients through the process. Tips for administration:
During and after each Spravato treatment session, you will be closely monitored for any potential side effects or adverse reactions. The healthcare provider will observe you for at least two hours after administering the medication to ensure your safety. During this time patients will be encouraged to engage in Cognitive Behavioral Therapy exercises to improve mental health symptoms. Cognitive Behavioral Therapy has many benefits and can improve treatment outcomes when combined with Spravato. Cognitive Behavioral Therapy: Cognitive Behavioral Therapy (CBT) is a widely used and evidence-based form of psychotherapy that focuses on the relationship between thoughts, emotions, and behaviors. It has been found effective in treating a range of mental health conditions. CBT helps individuals become aware of their negative or distorted thoughts and beliefs, known as cognitive distortions. Through therapy, individuals learn to challenge and reframe these thoughts, replacing them with more realistic and balanced thinking patterns. CBT equips individuals with a wide range of coping skills and techniques that can be used in real-life situations. These skills include problem-solving, stress management, relaxation techniques, assertiveness training, and effective communication, enabling individuals to navigate challenges and improve their overall functioning. CBT is a focused therapy that helps individuals target specific problems and challenges they are facing. It provides practical tools and techniques to address these issues directly, helping individuals make tangible progress and achieve their desired outcomes. CBT is known for its effectiveness in preventing relapse in many mental health conditions. By learning to recognize early warning signs and applying coping skills, individuals can better manage stressors and minimize the likelihood of a recurrence of symptoms. This process can lead to reduced emotional distress and improved coping mechanisms. It's important to note that while CBT has numerous benefits, different individuals may respond differently to therapy, and the effectiveness of CBT can vary. Conclusion: Spravato nasal spray, or esketamine, represents an innovative treatment option for individuals struggling with treatment-resistant depression and major depressive disorder with acute suicidal ideation or behavior. Its FDA approval has brought new hope for patients who have not responded well to traditional antidepressant therapies. While offering rapid symptom relief, it is crucial to consider the potential side effects associated with Spravato and to adhere to the REMS protocol to ensure safe and appropriate use.
0 Comments
|
AuthorImproving mental health through positive change. Archives
November 2023
Categories |
|
Monday 8am-12pm and 1pm-5pm
Tuesday 8am-12pm and 1pm-5pm Wednesday 8am-12pm and 1pm-5pm Thursday 8am-12pm and 1pm-5pm Friday 8am-12pm and 1pm-5pm |
Phone: 346-202-7570
Fax: 346-202-7571 Address: 719 Sawdust Road Ste 210 Spring, TX 77380 |

 RSS Feed
RSS Feed